Pocket PEA (Rapid Screening Chlorophyll Fluorometer)
Rapid screening continuous excitation chlorophyll fluorimeter
OVERVIEW
The Pocket PEA chlorophyll fluorimeter is suitable for teaching, research and a wide variety of commercial applications. The robust yet compact hand-held design provides ease of use and reliable operation.
Samples are conveniently dark adapted prior to measurement using the leafclips supplied. Single-button operation fully automates the complete measurement process from data capture through to calculation and display of the key and Performance Index (
) chlorophyll fluorescence parameters.
The rapid 1-second measurement capability and 200 measurement memory capacity make the Pocket PEA an invaluable tool in large-scale plant screening programs.
The chlorophyll fluorescence signal received by the sensor during recording is digitised in the control unit using a fast 16-bit analogue/digital converter ensuring excellent precision and repeatability of results. The fluorescence signal is digitised at different rates dependent upon the different phases of the induction kinetic. Initially, data is sampled at 10µs intervals for the first 300 µseconds. This provides excellent time resolution of and the initial rise kinetics. The time resolution of digitisation is then switched to slower acquisition rates as the kinetics of the chlorophyll fluorescence signal slow. This process allows optimum resolution of the overall measurement whilst minimising the size of the data set and thus maximising memory capacity.
 Bluetooth® wireless transfer conveniently allows records to be transferred in the field from the Pocket PEA to a suitable Bluetooth® enabled PC for detailed review and analysis using our custom Windows® PC software.
Bluetooth® wireless transfer conveniently allows records to be transferred in the field from the Pocket PEA to a suitable Bluetooth® enabled PC for detailed review and analysis using our custom Windows® PC software.
The Pocket PEA optical interface is mounted directly on to the front of the Pocket PEA control unit. It consists of a single high-intensity focused LED which is positioned vertically above the sample and provides up to 3,500 µmol m-2 s-1 intensity with a peak wavelength of 627nm at the sample surface. The light emitted from the LED is filtered using an NIR filter to block any infrared content which could be seen by the detector (known as optical breakthrough). An optical feedback circuit monitors and corrects changes in the output intensity of the LED which are caused by internal heat build-up in the LED itself. The circuit also compensates for intensity changes caused by variation in ambient temperature.
The detector is a highly sensitive PIN photodiode and associated amplifier circuit. The optical design and filtering ensure that it responds maximally to the longer-wavelength fluorescence signal and blocks the reflected shorter-wavelength LED light used as the source of illumination. The entire optical assembly is sealed behind a clear glass window which creates a barrier against moisture and dirt which are inherent problems for field-based instruments.
The latest lithium polymer battery technology ensures a full day of field usage and the convenience of rapid (<4hrs) recharge to full capacity using either the mains charger provided or an optional 12V DC vehicle charger.
Features
-
Ultra-portable chlorophyll fluorescence measurement system
-
Rapid screening capability with single-button operation
-
On-board storage of up to 200 full data sets
-
Automatic calculation of parameters including Fv/Fm & OJIP analysis
-
Robust enclosure with sealed, high-intensity optics
-
100kHz sampling frequency with 16-bit resolution
-
Bluetooth® wireless data transfer as standard
-
Powerful Windows® data transfer & analysis software included
System Components
Pocket PEA is supplied with the following components:
- PPEA: Pocket PEA control unit
- PPEA/LC: Pocket PEA leafclips x 20
- Protective carry bag
- USB drive containing PEA+ software and manuals.
Leafclips & Sample Dark Adaptation
Continuous excitation fluorescence systems like Pocket PEA, Handy PEA+ and M-PEA rely on the use of a suitable leafclip system with 2 functions. Firstly, the leafclip shields the fluorescence detector from ambient light which would otherwise “blind” the sensor due to the comparatively high levels of red/infrared light within the same waveband as the fluorescence itself. Secondly, the leafclip pre-conditions or dark adapts a section of the sample prior to the measurement.
Any measurement of the maximum photochemical efficiency of Photosystem II () requires the sample to be fully dark adapted prior to measurement. During dark adaptation, all reaction centers within the sample are fully oxidised making them available for photochemistry and any latent chlorophyll fluorescence yield is quenched. This process takes a variable amount of time and depends upon plant species, light history prior to the dark transition and whether or not the plant is stressed. Typically, 15 – 20 minutes may be required to dark adapt effectively.
Dark adaptation leafclips are constructed from plastic making them small and lightweight. The locating ring (which interfaces with the fluorimeter sensor) is positioned over the required area of the sample and has a central 4mm diameter hole which is covered using a shutter-plate. During measurement, this shutter slides back to expose the dark adapted sample to the focused LEDs and fluorescence detector. Pocket PEA leafclips have a black-coloured locating ring whereas Handy PEA+ and M-PEA leafclips have a white locating ring with a silvered underside which reflects incident light and minimises the build-up of heat on the sample. This ensures that the measurement is unaffected when measuring in high ambient light conditions.
PEA+ Software
PEA+ is a multi-function Windows® program supplied with Pocket PEA and Handy PEA+ for system configuration, data acquisition and post-measurement analysis.
Several different data presentation techniques have been combined in order to effectively demonstrate subtle differences in the fluorescence signature of samples which could be indicative of stress factors affecting the photosynthetic efficiency of the plant. Data may be presented in graphical, tabulated or radial plots which can all be tailored to display any number of the 58 parameters measured by both Pocket PEA and Handy PEA+. Transferred data may be exported to CSV format for further statistical analysis in external software packages.
PEA+ allows enhanced configuration of the Handy PEA+ via the Protocol Editor feature. Protocols may be defined to include single or multiple-measurement assays with optional pre-illumination periods which can then be uploaded to the memory of Handy PEA+ via USB communications. The use of protocols ensures maximum reproducibility of results during field applications involving large-scale screening away from a laboratory environment.
PEA+ will run on all supported Microsoft® operating systems.
Common Continuous Excitation Fluorescence Parameters Measured
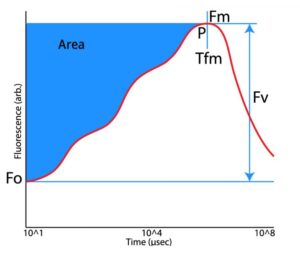
– Represents emission by excited chlorophyll a molecules in the antennae structure of Photosystem II. The true
level is only observed when the first stable electron acceptor of Photosystem II called
is fully oxidised. This requires thorough dark adaptation.
– The maximum fluorescence value obtained for a continuous light intensity. This parameter may only be termed as maximal if the light intensity used is fully saturating and the electron acceptor
is fully reduced.
– Indicates the variable component of the recording and relates to the maximum capacity for photochemical quenching. Calculated by subtracting the
value from the
value (
).
– An indication of the maximum quantum efficiency of Photosystem II and widely considered to be a sensitive indicator of plant photosynthetic performance. Presented as a ratio between 0 and 1, healthy samples typically achieve a maximum
value of approx. 0.85. Values lower than this will be observed if a sample has been exposed to some type of biotic or abiotic stress factor which has reduced the capacity for photochemical quenching within PSII.
is presented as a ratio of variable fluorescence (
) over the maximum fluorescence value (
) and is calculated as
.
– Indicates the time at which the maximum fluorescence value (
) was reached. May be used to indicate sample stress which causes the
to be reached much earlier than expected.
– The area above the fluorescence curve between
and
is proportional to the pool size of the electron acceptors
on the reducing side of Photosystem II. If electron transfer from the reaction centers to the quinone pool is blocked (such as is the mode of action of the photosynthetically active herbicide DCMU), the area will be dramatically reduced.
Time Marks Parameters
The PEA Plus and M-PEA Plus software packages extract chlorophyll fluorescence values from the recorded data from Handy PEA+, Pocket PEA and M-PEA chlorophyll fluorimeters at 5 pre-defined Time Marks. The times are:
= 50 microseconds
= 100 microseconds
= (
step) 300 microseconds
= (
step) 2 milliseconds
= (
step) 30 milliseconds
Chlorophyll fluorescence values at these Time Marks are used to derive a series of further biophysical parameters, all referring to time base 0 (onset of fluorescence induction), that quantify the photosystem II behaviour for (A) The specific energy fluxes (per reaction center) for:
- Absorption (
)
- Trapping (
)
- Dissipation (
)
- Electron transport (
)
and (B) the flux ratios or yields:
- Maximum yield of primary photochemistry (
)
- Efficiency (
) with which a trapped exciton can move an electron into the electron transport chain further than
- Quantum yield of electron transport (
)
The concentration of active PSII reaction centers per excited cross section () is also calculated.
Performance Index Parameters (OJIP Analysis)
The Performance Index () is essentially an indicator of sample vitality. It is an overall expression indicating a kind of internal force of the sample to resist constraints from outside. It is a Force in the same way that redox potential in a mixture of redox couples is a force. Exactly the
is a force if used on a log scale. Therefore we say:
is derived according to the Nernst equation. It is the equation which describes the forces of redox reactions and general movements of Gibbs free Energy in biochemical systems. Such a force (or potential = force) is defined as:-
where is the fraction of a partner in the reaction
to
. Therefore:
and if you now convert to:
or for redox reactions
Now the total potential in a mixture is the sum of the individual potentials or:
….etc
In our case (on an absorption basis or on a chlorophyll basis) has three components:
The first component shows the force due to the concentration of active reaction centers
therefore:
is a parameter of the
test and it is related to the force generated by the RC concentration per antenna chlorophyll.
The second component is the force of the light reactions, which is related to the quantum yield of primary photochemistry:
The driving force of the light reactions is therefore:
The third component is the force related to the dark reactions (after ). These are normal redox reactions in the dark.Expressed by the
test as:
Where = relative variable fluorescence at 2 ms or at the step
therefore:
Therefore the force of the dark reactions is:
Now all three components together make:
or without log
or in fluorescence terms:
All Parameters Measured
- OJIP data:
- Normalised data:
- Specific fluxes:
- Apparent fluxes per CSo:
- Partial performances:
- Time marks:
- Partial areas:
to
to
to
to
to
to
to
- Slopes & integrals:
- Yield = flux ratios:
- Apparent fluxes per CSm:
- Total performance, driving force & rates:
- User parameter:
- 3 User-entered values
Technical Specifications
- Dimensions: 175mm (l) x 75mm (w) x 35mm (d)
- Weight 250g
- Communications: Bluetooth® wireless communications
- Operating conditions:
- 0°C – 40°C
- Non-condensing humidity.
- Battery: Environmentally friendly (0% lead, cadmium mercury) lithium polymer 3.7V, 570 mAhr
- Battery charger:
- Integral switch mode charger
- Input voltage 100V – 240V at 50Hz – 60Hz
- Output voltage 12V DC
- Output current 3 amps.
- Display: 2-line x 12-character LCD display
- Illumination:
- Optically stabilised, focused, ultra-bright red LED with NIR short-pass cut-off filters
- Peak wavelength 627nm.
- Max. intensity at leaf surface: Up to 3,500 μmol m-2 s-1
- Detector: Fast-response PIN photodiode with RG9 long-pass filter
- Electronics:
- High-performance 16-bit microcontroller
- 16-bit resolution A/D 10 μsecond acquisition rate
- 8-bit DAC for light source control
- real-time clock.
- Record Length: 1, 3 or 10 seconds
- Memory:
- 512 Kbits non-volatile memory
- Sufficient for up to 200, 10-second duration recordings with full trace data.